15% off £20
What is THC? How does it differ to CBD?

There is increasing interest in the growing availability of products extracted from the cannabis plant. Whilst cannabidiol (CBD) oil is growing in popularity, the first molecule that many think of when it comes to the cannabis plant is tetrahydrocannabinol (THC). Despite some differences, there are some fundamental differences between CBD, which we shall explore during this article.
What is THC?
THC is one of the key chemical components from the flower of the Cannabis plant and was first identified in 1964 by Israeli-based chemist Raphael Mechoulam.1 It is most well-known for being the psychotropic component of the plant which is responsible for an associated euphoria when administered at high concentrations recreationally.
THC belongs to a group of compounds known as cannabinoids. There are over 140 cannabinoids that have been identified to date from the cannabis flower.2 These cannabinoids share similarities in structure to THC and binds at Cannabinoid Receptors in the human body.3 The most well-known cannabinoid apart from THC is CBD.


What does THC stand for?
THC is an abbreviation of tetrahydrocannabinol. More specifically it is typically used to refer to the compound (−)-trans-Δ9-tetrahydrocannabinol. However, there are several other compounds which either share a similar chemical structure as (−)-trans-Δ9-tetrahydrocannabinol or have similar properties, which THC is occasionally used to refer to:
- Dronabinol is a synthetic version (−)-trans-Δ9-tetrahydrocannabinol used in pharmaceuticals. It is licensed in the United States for use in treating anorexia associated with acquired immune deficiency syndrome (AIDS) and chemotherapy-induced nausea and vomiting. However, it is not licensed in the United Kingdom and is not available for prescription.
- Delta-8-tetrahydrocannabinol, which has a small change in structure to (−)-trans-Δ9-tetrahydrocannabinol, however, it is not found in large concentrations within the cannabis plant.
- Nabilone is a synthetic mimic of (−)-trans-Δ9-tetrahydrocannabinol and is a licensed therapy for chemotherapy-induced nausea and vomiting in the United Kingdom.
Throughout the remainder of this article THC will be used to refer to (−)-trans-Δ9-tetrahydrocannabinol, unless said otherwise.
What is the difference between CBD and THC?
THC and CBD each consist of 21 carbon atoms, 30 hydrogen atoms and 2 oxygen atoms. Whilst they also share similarities in shape, their subtle differences result in contrasting effects within the human body.
Both compounds interact with the body’s own endogenous cannabinoid system – called the endocannabinoid system. This is made up of neurotransmitters which resemble cannabinoids found in the cannabis plant, called endocannabinoids, and Cannabinoid Receptors.

The endocannabinoid system plays a regulatory role in modulating the release of other molecules and receptors. It has subsequently been referred to as ‘nature’s dimmer switch’.4
Cannabinoid Receptor 1 (CB1) is predominantly found in the central nervous system and when active results in dampening of transmission between different neurons. 5
Cannabinoid Receptor 2 (CB2) is found most within cells that are components of the immune system.5
THC activates both CB1 and CB2 receptors. It is through its actions at CB1 receptors it is thought to regulate emotions, coordination, and cognition.6
CBD has a complex role at Cannabinoid Receptors. CBD inhibits the breakdown of endocannabinoids, leading to increased endocannabinoids available to activate Cannabinoid Receptors.6
In addition, there is an extended system of other receptors and proteins which are affected by both THC and CBD, which lead to their individual effects.7
Figure 1 – The Endocannabinoid System and Receptors (Image reused with permission of the Sapphire Institute for Medical Cannabis Education)
THC versus CBD
Hopefully by the time you have reached this section of the article you will have an understanding of what THC is and how it differs to CBD. Let’s now compare THC and CBD, starting with looking at the structure.
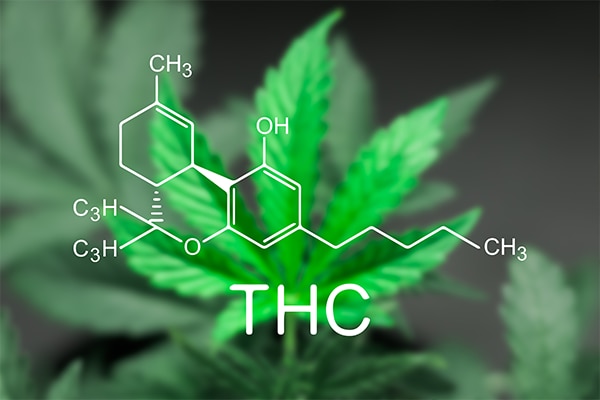
What is the structure of THC?
THC contains 21 carbon atoms, 30 hydrogen atoms and 2 oxygen atoms. This is the same as CBD and subsequently they share the same chemical formula (C21H30O2).
As seen in the figure below all cannabinoids, not just THC and CBD, share a dibenzopyran ring and a hydrophobic alkyl chain.
However, variations beyond this are what result in the individual properties of THC, CBD and the less prevalent cannabinoids.
Figure 2 – Structure of tetrahydrocannabinol (THC), cannabidiol (CBD) and other cannabinoids from the cannabis plant
What does THC do?
THC activates both CB1 and CB2 receptors. Through its actions on cannabinoid receptors within the central nervous system THC regulates neurotransmission resulting in psychotropic effects, including regulation of emotions, motor coordination, and cognition.8
THC also acts as an agonist as peripheral pain receptors, called transient receptor potential (TRP) channels. At appropriate doses THC can desensitise these receptors and reduce peripheral pain inputs.9
The main action of CBD, however, is to inhibit the breakdown of the endocannabinoid called anandamide which activates the CB1 receptor directly.10
What does THC look like?
THC and CBD can be found in several different forms. First and foremost, they are found within dried flower from the cannabis plant. Subsequently the flower can be processed to extract cannabinoids and create oils, capsules, lozenges, and other ways to administer THC and CBD.
Medical use of THC:
THC when prescribed in medical settings is normally started at the lowest possible dose. Increasing the dose at a fast rate may increase the incidence of adverse events.
CBD when used in medical settings is typically administered in higher doses compared to THC as it is better tolerated at higher doses. The amount of CBD can vary according to medical indication. For epilepsy, for example, the typical dose described in randomised controlled trials is between 10-20 mg/kg/day.11 However, in chronic pain the doses prescribed are typically lower.


What are the benefits of THC?
THC is used as a component of treatment programmes utilising cannabis-based medicinal products.
In the UK cannabis-based medicinal products can be initiated as a treatment for conditions where there is sufficient clinical evidence by a specialist medical consultant, supported by a multi-disciplinary team. The largest group of patients being prescribed cannabis-based medicinal products containing THC is those with chronic pain.12
Evidence from the UK Medical Cannabis Registry has demonstrated improved quality of life, sleep, and pain severity/interference at 6 months in those prescribed cannabis-based medicinal products for chronic pain.13
Similar analysis of patients completing six months of treatment for anxiety demonstrated a significant reduction from moderate to mild anxiety according to validated assessment tools.6
However, despite this there is insufficient randomised controlled trial data to suggest THC outside of select conditions where there has been insufficient response to first line treatments.
Epidyolex ® is an isolated CBD preparation which is licensed as an add-on therapy for treatment-resistant epilepsy in Dravet and Lennox-Gastaut syndromes. Otherwise, cannabis-based medicinal products that contain CBD, like THC, are only otherwise indicated where there has been insufficient response to first line treatments. CBD oils and other products available over the counter are unlikely to have been manufactured to pharmaceutical-grade quality and therefore are not recommended as treatments for health conditions.
What are the side effects of THC?
The side effects of THC and CBD can vary widely depending on the person, the products used, and the dose of each product. According to existing evidence on maximum tolerated doses of THC (120mg total dose) and CBD (120mg total dose) containing cannabis-based medicinal products the side effects can differ from mild to serious and can include:
- feeling drowsy or sleepy
- decreased appetite
- diarrhoea
- fever
- feeling tired
- vomiting or nausea
- lack of energy or giddy
- blurred vision
- eating more than usual
- difficulty speaking
- change of taste or dry mouth
- constipation/diarrhoea
- cold, sore throat/mouth
- respiratory tract infections (pneumonia, bronchitis)
- blood tests showing increases in levels of certain liver enzymes or damage to the liver (signs include: abdominal pain, unexplained nausea and malaise, darkening of urine or jaundice)
- shaking, of the body or parts of it
- feeling bad-tempered (irritable, aggressive)
- difficulty sleeping
- cough
- rash
- increased appetite or weight loss
- sialorrhea (drooling)
- urinary tract infection
- abnormal behaviours or agitation
- loss of balance or falling over
- fainting
- changes in pulse rate, heart rate or blood pressure
- tummy pain
- mouth or teeth changing colour
Does THC get you high?
When taken recreationally, cannabis, is most commonly taken with the intention of achieving a ‘high’ or euphoria. Within the cannabis plant, it is THC which is responsible for this effect. As such recreational strains often contain higher concentrations of THC and are consumed in greater quantitates.
When utilised as a component of cannabis-based medicinal products, this euphoria is an unwanted adverse event. To mitigate this THC is often initiated at low levels and quantities, then increased slowly so the patient builds tolerance to this specific effect.
CBD does not cause a ‘high’ because it does not directly activate the CB1 receptors which are responsible for this effect.14
Is THC legal?
As per the Misuse of Drugs Act 1971, THC is a controlled drug and therefore it is illegal to possess, supply, produce, or import/export.
In November 2018, a change in the law allowed for the prescribing of unlicensed cannabis-based medicinal products in certain circumstances, by specialists on the GMC register, including those which contain THC.
For a product to be classified as a cannabis-based medicinal product it must meet the following requirements:
- The product is or contains cannabis, cannabis resin, cannabinol or a cannabinol derivative
- It is produced for medicinal use in humans; and
- It is a product that is regulated as a medicinal product, or an ingredient of a medicinal product.
‘Pure’ CBD based products that contain less than the statutory limit of THC are not controlled drugs and therefore are subject to fewer restrictions.
Does THC show up on a drug test?
THC can be detected using drug tests including urine, saliva, hair and blood samples. THC has a long half-life and can be present in samples for 30 days or greater.15 In hair samples it can be present up to 90 days following consumption.16
The length of time that it continues to stay in the body or show up on a drug test depends on:
- Body fat of the individual
- Speed of metabolism by the liver
- Frequency and quantity of consumption
- Sensitivity and type of drug test


There are no drug tests for CBD as it is not a controlled drug. However, many CBD products will contain trace amounts of THC. It is therefore possible depending on the composition and quality of a CBD product that it may result in a positive drug test result for THC.
What is THC oil?
As THC is lipid soluble, to create ways to consume the compound orally or under the tongue it has been added to oil-based solutions.
When used within cannabis-based medicinal products, this has the benefit of improved control of individual dose. Moreover, it leads to slower, more sustained release of its clinical effects.
Is THC oil legal?
THC oil is only legal within the confines of the Misuse of Drugs Act if it is a component of a cannabis-based medicinal product. Any prescription must be initiated by a doctor on the GMC specialist register, trained to treat the specific condition for which the patient is receiving treatment. The person must have also previously tried licensed medical therapies as in the UK THC oil is an unlicensed medicine.
Any consumption, possession, supply, produce, or import/export of THC outside of this specific situation is illegal.


CBD oil, that contains less than the regulated amounts of THC, is not subject to similar legal restrictions and can be available as a wellness product, within food and drink, or as a component of cannabis-based medicinal products.
Can you buy THC products?
THC products are only available for purchase if you are in receipt of a prescription for a cannabis-based medicinal product. This is determined by medical need.
As previously mentioned, any possession, supply or purchasing of THC oil is strictly prohibited.
CBD products produced according to good manufacturing practices are determined as medicinal quality and are only available via a prescription. These can only be initiated by a hospital consultant that specialises in the condition, supported by a multidisciplinary team. These are dispensed via specials pharmacies.
Other CBD products available for purchase must conform to novel foods regulations. This helps ensure their safety for mass use, but the products are not manufactured to a pharmaceutical standard to enable them to be used in the treatment of specific conditions. These can be purchased over the counter without a prescription.
Conclusions
Whilst THC and CBD may share the same basic chemical makeup in terms of carbon, hydrogen and oxygen atoms. The structure of each molecule results in markedly different effects on the human body. Subsequently, this has affected how each is consumed and the regulations which govern access and use.
THC is most well-known for its psychotropic effects in recreational users, however there is a growing body of evidence for its therapeutic effects within cannabis-based medicinal products. However, this evidence is still insufficient to recommend its use outside of those who have failed first-line treatment for distinct medical conditions.


CBD, however, doesn’t cause any ‘high’ following consumption and this has likely led to fewer restrictions governing access and use. As a medicinal product it now has a license in the UK as an add-on treatment for certain childhood epilepsy syndromes. There is burgeoning evidence in other conditions as well. CBD products not obtained via prescription, however, have little to no clinical research to evaluate their effects on specific conditions or general health.
- https://pubs.acs.org/doi/10.1021/ja01062a046
- https://pubmed.ncbi.nlm.nih.gov/16402100/
- https://pubmed.ncbi.nlm.nih.gov/30001569/
- https://pubmed.ncbi.nlm.nih.gov/30360776/
- https://pubmed.ncbi.nlm.nih.gov/30139415/
- https://pubmed.ncbi.nlm.nih.gov/34937473/
- https://pubmed.ncbi.nlm.nih.gov/31831863/
- https://pubmed.ncbi.nlm.nih.gov/30522595/
- https://pubmed.ncbi.nlm.nih.gov/34853533/
- https://pubmed.ncbi.nlm.nih.gov/25666611/
- https://pubmed.ncbi.nlm.nih.gov/32592183/
- https://pubmed.ncbi.nlm.nih.gov/33988306/
- https://pubmed.ncbi.nlm.nih.gov/34937473/
- https://pubmed.ncbi.nlm.nih.gov/34467598/
- https://pubmed.ncbi.nlm.nih.gov/24185550/
- https://pubmed.ncbi.nlm.nih.gov/27296783/
















